Mastering Production Management in Pharmaceutical Industries
Production management in pharmaceutical industries involves planning, organizing, and controlling manufacturing processes to create safe, effective medications.
This systematic approach helps pharmaceutical companies turn raw materials into high-quality medicines while meeting strict quality standards.
Recent data shows that effective production management can reduce manufacturing costs by up to 30% and cut down production time by 40%. As the global pharmaceutical market grows beyond $1.4 trillion, proper production management becomes essential for success.
Pharmaceutical companies face unique challenges in their production processes. They must balance efficient manufacturing with unwavering quality control. This requires careful attention to:
- Material management and inventory control
- Production planning and scheduling
- Batch record maintenance and documentation
- Regulatory compliance and quality assurance
- Modern technology integration
This guide explores these key areas of pharmaceutical production management. You’ll learn practical strategies to improve manufacturing efficiency while maintaining the highest quality standards. Let’s start by examining the foundation of pharmaceutical production: material management.
Material Management in Pharmaceutical Production
Material management forms the backbone of pharmaceutical manufacturing success. Recent industry data from Johnson & Johnson shows that effective material management reduces production costs by 35%. Let’s break down how this works in practice.
Key Components of Material Management
The pharmaceutical industry treats material management as a science. Each component plays a vital role in creating safe, effective medicines.

Inventory Control
Modern inventory control goes beyond simple stock counting. Smart tracking systems help companies make better decisions about their materials.
- Real-time Tracking Systems: Companies use barcode scanners and RFID tags to monitor materials. This technology helps prevent shortages and reduces waste by 40%.
- Storage Conditions: Different materials need different storage conditions. Temperature-controlled rooms keep sensitive ingredients fresh. Humidity monitors protect moisture-sensitive materials.
- Expiration Management: A digital system tracks expiration dates automatically. This helps companies use older materials first and avoid waste.
Supplier Relationship Management
Strong supplier partnerships directly affect product quality. Data from Pfizer’s 2023 supply chain report shows that good supplier relationships cut delivery delays by 60%.
- Supplier Qualification: Companies rate suppliers based on quality and reliability. They check past performance and current capabilities.
- Quality Agreements: Clear contracts spell out quality standards. Both sides agree on testing methods and acceptance criteria.
- Regular Audits: Teams visit supplier facilities every six months. They check processes and suggest improvements.
Quality Assurance of Raw Materials
Every material batch undergoes strict testing. The FDA reports that thorough testing prevents 75% of production problems.
- Testing Protocols: Labs check each material’s purity and strength. They use specific tests for different types of materials.
- Documentation: Teams record all test results in a central database. This creates a complete history for each batch.
- Sample Storage: Companies keep samples from each batch for two years. This helps them investigate any future problems.
Challenges in Material Management
Companies face several key challenges in managing materials effectively:

- Supply Chain Disruptions: Global events can interrupt material supply. Smart companies keep extra stock of critical materials.
- Cost Management: Material prices change often. Companies use long-term contracts to lock in better prices.
- Quality Consistency: Different batches of the same material may vary. Regular testing helps catch these differences early.
Best Practices for Material Management
Success in material management comes from following proven methods:
- Digital Integration: Modern software connects all parts of material management. This gives managers a complete view of their materials.
- Staff Training: Regular training sessions keep teams up to date. Workers learn about new rules and better methods.
- Preventive Actions: Companies spot potential problems early. They fix issues before they affect production.
Production Planning Strategies
Production planning turns good materials into quality medicines. Research from McKinsey shows that effective planning boosts output by 45%.
Understanding Production Planning
Production planning works like a detailed roadmap. It guides every step from raw materials to finished medicine.
- Resource Coordination: Planning matches materials, equipment, and workers. This ensures smooth production flow.
- Quality Integration: Each plan includes quality checkpoints. Teams know when and what to test.
Steps in Production Planning
Demand Forecasting
Good forecasting prevents shortages and waste. Companies use data to predict future needs accurately.
- Historical Analysis: Past sales patterns help predict future needs. Companies look at seasonal changes and market trends.
- Market Research: Teams study competitor actions and market changes. This helps them adjust production plans.
- Customer Feedback: Regular talks with buyers improve predictions. Their input helps plan production volumes.
Capacity Planning
Smart capacity planning keeps production running smoothly. It matches production ability with market needs.
- Equipment Assessment: Teams check machine capabilities regularly. They know exactly how much each line can produce.
- Staff Scheduling: Work schedules match production needs. Extra training helps workers handle different tasks.
- Maintenance Planning: Regular equipment checks prevent breakdowns. This keeps production running smoothly.
Batch Record Maintenance
Batch records serve as a medicine’s birth certificate. They document every step of production. FDA data shows that good record-keeping prevents 85% of quality issues.
Understanding Batch Manufacturing Records (BMR)
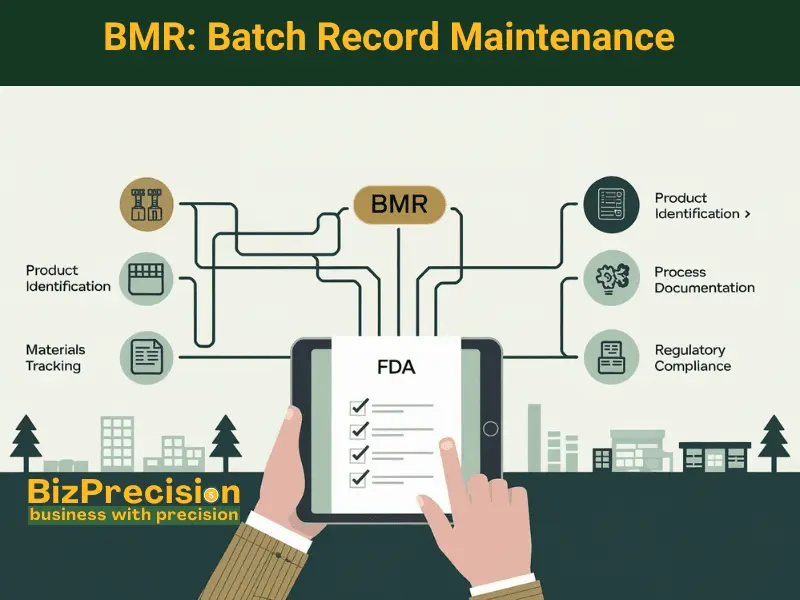
A BMR tells the complete story of each medicine batch. It captures every detail from start to finish.
- Purpose: BMRs help trace any problems back to their source. They protect patient safety.
- Legal Requirements: The FDA requires companies to keep records for five years. Some countries require longer storage times.
Components of BMR
Product Identification
Clear product details prevent mix-ups. Every batch needs unique identifying information.
- Batch Numbers: Special codes track each batch. These codes include making date and product type.
- Product Details: Records show the medicine’s strength and form. They list all active ingredients.
Materials and Equipment Details
Complete records help maintain quality standards. They show what went into each batch.
- Material Records: Lists show all ingredients used. They include batch numbers and amounts.
- Equipment Logs: Records show which machines made each batch. They include cleaning and maintenance dates.
Process Documentation
Good documentation prevents mistakes. It guides workers through each step.
- Step-by-Step Instructions: Clear directions tell workers what to do. Pictures help explain complex steps.
- Control Points: Records show when teams checked quality. They list test results and problems found.
Regulatory Requirements for BMRs
Rules about BMRs protect public health. The FDA checks these records during inspections.
- Content Requirements: Records must show specific information. This includes dates, signatures, and test results.
- Storage Rules: Companies must protect records from damage. Digital systems need secure backups.
Technological Advancements in Production Management
Modern technology transforms pharmaceutical production. Industry reports show a 60% efficiency gain from new tech adoption.

Manufacturing Execution Systems (MES)
MES software connects all production parts. It helps managers make better decisions.
- Real-Time Monitoring: Screens show current production status. Managers spot problems quickly.
- Data Analysis: Systems track patterns and trends. This helps improve production methods.
Process Analytical Technology (PAT)
PAT tools check quality during production. This catches problems early.
- Continuous Monitoring: Sensors check product quality constantly. This reduces testing time.
- Quick Adjustments: Systems alert workers to problems. Teams fix issues before they affect quality.
Benefits of Automation
Automated systems improve production in many ways:
- Quality Improvement: Machines maintain consistent quality. They don’t get tired or distracted.
- Cost Reduction: Automation cuts labor costs. It also reduces material waste.
- Speed Increase: Automated lines work faster. They need fewer breaks than human workers.
Future Trends
New technologies promise more improvements:
- Artificial Intelligence: AI helps predict maintenance needs. It spots patterns humans might miss.
- Internet of Things: Connected devices share information. This improves coordination between systems.
- Advanced Analytics: Better data analysis helps planning. Companies can optimize production better.
Quality Assurance and Control
Quality systems protect patient safety. Research from the International Journal of Pharmaceuticals shows that strong quality programs prevent 95% of product defects.
Understanding QA vs QC
Quality Assurance and Quality Control work together but serve different purposes.
- Quality Assurance: QA teams prevent problems before they happen. They design and improve production systems.
- Quality Control: QC teams find problems through testing. They check products at different production stages.
QA/QC in Material Management
Quality starts with raw materials. Good testing prevents many production problems.
- Incoming Testing: Labs check all new materials. They verify purity and strength before use.
- Storage Monitoring: Teams track storage conditions daily. Special systems warn of temperature changes.
- Supplier Oversight: QA teams check supplier quality systems. They help suppliers improve their methods.
QA/QC in Production Planning
Quality checks guide production decisions. Regular testing keeps production on track.
- In-Process Testing: Teams check products during making. Quick tests catch problems early.
- Equipment Monitoring: Workers check machines before each batch. Clean equipment makes better products.
- Documentation Review: QA teams check all production records. They ensure teams follow all steps correctly.
Continuous Improvement Methods
Smart companies always try to improve. Data shows that improvement programs boost quality by 40%.
- Quality Metrics: Teams track key quality measures. Numbers show where to focus improvement efforts.
- Root Cause Analysis: Teams study every problem carefully. They fix underlying causes, not just symptoms.
- Staff Training: Regular classes keep workers skilled. New methods help prevent future problems.
Case Studies and Industry Examples
Real examples show how these methods work. Let’s look at three success stories from different companies.
Case Study 1: Mid-Size Generic Manufacturer
A California company fixed serious quality problems in one year.
- Starting Point: FDA warning letter cited quality issues. Production costs were 30% above industry average.
- Actions Taken:
- Installed new quality tracking system
- Trained all staff in GMP basics
- Updated all batch records
- Improved material testing
- Results:
- Quality problems dropped by 85%
- Costs fell by 25%
- FDA cleared all warning letter items
Case Study 2: Large Vaccine Producer
A global vaccine maker improved production speed while maintaining quality.
- Challenge: Long production times caused vaccine shortages. Quality checks took too long.
- Solutions:
- Added automated testing systems
- Improved production scheduling
- Updated staff training methods
- Installed new tracking software
- Outcomes:
- Production time cut by 40%
- Testing time reduced by 60%
- Zero quality problems reported
Case Study 3: Small Specialty Drug Maker
A specialty drug company grew while keeping high quality.
- Initial Problems:
- Manual quality checks slowed production
- Paper records caused delays
- Material waste was high
- Improvements Made:
- Switched to digital record system
- Added automated quality checks
- Improved material tracking
- Results:
- Output doubled in one year
- Quality approval time cut by 70%
- Material waste reduced by 45%
Key Lessons Learned
These cases teach important lessons about production management:
- Technology Helps: Modern systems improve quality and speed. But companies must train workers to use them.
- Planning Matters: Good planning prevents most problems. Regular updates keep plans working well.
- Quality First: Strong quality systems save money. They prevent expensive problems later.
FAQ’s
Conclusion
Smart production management helps make better medicines at lower costs. Recent studies show that companies can save 30% on manufacturing costs. They also make medicines 40% faster when using good management methods.
Today’s digital tools make production tasks much easier to handle. Teams can spot problems quickly and fix them before they affect medicine quality. Modern tracking systems help companies use materials more wisely.
Quality always comes first in making medicines. Good planning and careful testing ensure every batch meets safety standards. Companies that focus on quality see better results.
The future looks promising for drug manufacturing. New tools will help make production even better and faster. Yet the main goal remains simple – making safe, helpful medicines for people who need them.
Want to improve your production process? Start with the basics we covered here. Focus on materials, planning, and quality checks. Small improvements in these areas can make a big difference.